THYMOSIN ALPHA 1
thymosin A1 bovine
CAS: 62304-98-7
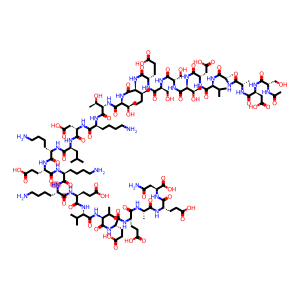
Molecular Formula: C129H215N33O55
THYMOSIN ALPHA 1 - Names and Identifiers
| Name | thymosin A1 bovine |
| Synonyms | Zadaxin ZADAXIN Thymotin Thymosin α1 Thymalfasin Thymosinalpha1 THYMOSIN APHA 1 UNII-W0B22ISQ1C THYMOSIN ALPHA 1 Thymosin alpha 1 thymosin A1 bovine Thymosin α1 Acetate Thymosin alpha1 (ox) Thymosin αAcetate salt THYMOSIN ALPHA1 BOVINE Thymosin alpha1 (human) Thymalfasin Acetate salt Thymosin alpha1 (cattle) Thymosin α 1 Acetate(Thymalfasin) N-Acetyl-L-seryl-L-alpha-aspartyl-L-alanyl-L-alanyl-L-valyl-L-alpha-aspartyl-L-threonyl-L-seryl-L-seryl-L-alpha-glutamyl-L-isoleucyl-L-threonyl-L-threonyl-L-lysyl-L-alpha-aspartyl-L-leucyl-L-lysyl-L-alpha-glutamyl-L-lysyl-L-lysyl-L-alpha-glutamyl-L-valyl-L-valyl-L-alpha-glutamyl-L-alpha-glutamyl-L-alanyl-L-alpha-glutamyl-L-asparagine L-Asparagine, N-acetyl-L-seryl-L-alpha-aspartyl-L-alanyl-L-alanyl-L-valyl-L-alpha-asparatyl-L-threonyl-L-seryl-L-seryl-L-alpha-glutamyl-L-isoleucyl-L-threonyl-L-threonyl-L-lysyl-L-alpha-aspartyl-L-leucyl-L-lysyl-L-alpha-glutamyl-L-lysyl-L-lysyl-L-alpha-glutamyl-L-valyl-L-valyl-L-alpha-glutamyl-L-alpha-glutamyl-L-alanyl-L-alpha-glutamyl- |
| CAS | 62304-98-7 |
| EINECS | 1592732-453-0 |
| InChI | InChI=1/C129H215N33O55/c1-18-59(10)98(159-114(201)76(36-42-91(181)182)146-120(207)83(53-164)154-121(208)84(54-165)155-127(214)99(63(14)166)160-118(205)80(51-94(187)188)152-123(210)95(56(4)5)156-104(191)62(13)135-102(189)60(11)137-115(202)78(49-92(183)184)151-119(206)82(52-163)138-66(17)169)125(212)161-101(65(16)168)128(215)162-100(64(15)167)126(213)147-70(30-22-26-46-133)109(196)150-79(50-93(185)186)117(204)149-77(47-55(2)3)116(203)142-69(29-21-25-45-132)108(195)144-73(33-39-88(175)176)110(197)141-67(27-19-23-43-130)106(193)140-68(28-20-24-44-131)107(194)145-75(35-41-90(179)180)113(200)157-97(58(8)9)124(211)158-96(57(6)7)122(209)148-74(34-40-89(177)178)111(198)143-71(31-37-86(171)172)105(192)136-61(12)103(190)139-72(32-38-87(173)174)112(199)153-81(129(216)217)48-85(134)170/h55-65,67-84,95-101,163-168H,18-54,130-133H2,1-17H3,(H2,134,170)(H,135,189)(H,136,192)(H,137,202)(H,138,169)(H,139,190)(H,140,193)(H,141,197)(H,142,203)(H,143,198)(H,144,195)(H,145,194)(H,146,207)(H,147,213)(H,148,209)(H,149,204)(H,150,196)(H,151,206)(H,152,210)(H,153,199)(H,154,208)(H,155,214)(H,156,191)(H,157,200)(H,158,211)(H,159,201)(H,160,205)(H,161,212)(H,162,215)(H,171,172)(H,173,174)(H,175,176)(H,177,178)(H,179,180)(H,181,182)(H,183,184)(H,185,186)(H,187,188)(H,216,217)/t59-,60-,61-,62-,63+,64+,65+,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,95-,96-,97-,98-,99-,100-,101-/m0/s1 |
THYMOSIN ALPHA 1 - Physico-chemical Properties
| Molecular Formula | C129H215N33O55 |
| Molar Mass | 3108.32 |
| Density | 1.360±0.06 g/cm3(Predicted) |
| Boling Point | 2899.7±65.0 °C(Predicted) |
| Solubility | It is recommended to reconstitute the lyophilized Thymosin a1 in sterile 18MΩ-cm H2O not less than 100 μg/ml, which can then be further diluted to other aqueous solutions. |
| Color | Sterile Filtered White lyophilized (freeze-dried) powder. |
| Storage Condition | −20°C |
| Stability | Lyophilized Thymosin a1 although stable at room temperature for 3 weeks, should be stored desiccated below -18°C. Upon reconstitution Thymalfasin should be stored at 4°C between 2-7 days and for futur |
| Use | Treatment of chronic hepatitis B; Immunopotentiator; For the treatment of hepatitis B, various types of chronic hepatitis and serious hepatitis, herpes, condyloma, viral keratitis |
| In vitro study | Thymalfasin has been shown to have efficacy in multiple experimental models of immune dysfunction, mainly, infectious diseases such as hepatitis (woodchuck) and influenza (mouse), and cancer such as melanoma (mouse) and colorectal carcinoma (rat) where thymalfasin has shown antitumor effects. |
| In vivo study | Thymalfasin has been shown to have efficacy in multiple experimental models of immune dysfunction, mainly, infectious diseases such as hepatitis (woodchuck) and influenza (mouse), and cancer such as melanoma (mouse) and colorectal carcinoma (rat) where thymalfasin has shown antitumor effects. |
THYMOSIN ALPHA 1 - Risk and Safety
| WGK Germany | 3 |
THYMOSIN ALPHA 1 - Standard
Authoritative Data Verified Data
This product is a chemically synthesized polypeptide consisting of twenty-eight amino acids. N-acetyl-L-seryl-La-aspartyl-L-alanyl-L-alanyl-L-alanyl-L-aspartyl-L-a-aspartyl-L-L-seryl-L-seryl-L-a-glutamyl-L-isoleucyl-L-susyl-L-susyl-L-lysyl-L-«-aspartyl-L-leucyl-L-lysyl-L-a-glutamyl-L-lysyl-L-lysyl-L-a-glutamyl-L-alanyl-L-N-alanyl-L-a-glutamyl-L-alanyl-L-a-glutamyl-L-a-L-asparagine. The thymalfasin content (C129H215N33O55) shall be 95.0% ~ 105.0% calculated as anhydrous and acetic acid-free.
THYMOSIN ALPHA 1 - Nature
Authoritative Data Verified Data
- This product is white or off-white powder or loose block.
- This product is dissolved in trifluoroacetic acid, slightly soluble in water, insoluble in acetonitrile and ether.
specific rotation
take this product, precision weighing, add 0.02mol/L phosphate buffer (pH 7.0 )(take potassium dihydrogen phosphate 2.72g, add 0. 116.4ml of lmol/L sodium hydroxide solution was dissolved, diluted to 1000ml with water), dissolved and quantitatively diluted to prepare a solution containing about 5mg per lml, which was determined according to law (General rule 0621), without acetic acid, the specific rotation is from one 100.0 ° to one 110.0 °.
THYMOSIN ALPHA 1 - Preparation solution concentration reference
| 1mg | 5mg | 10mg | |
|---|---|---|---|
| 1 mM | 0.322 ml | 1.609 ml | 3.217 ml |
| 5 mM | 0.064 ml | 0.322 ml | 0.643 ml |
| 10 mM | 0.032 ml | 0.161 ml | 0.322 ml |
| 5 mM | 0.006 ml | 0.032 ml | 0.064 ml |
THYMOSIN ALPHA 1 - Differential diagnosis
Authoritative Data Verified Data
- take about 2mg of this product, add 1 ml of water to dissolve, and add 1 ml of Alkaline copper tartrate test solution, which shows blue and purple color.
- in the chromatogram recorded under the content determination item, the retention time of the main peak of the test solution should be consistent with the retention time of the main peak of the reference solution.
THYMOSIN ALPHA 1 - Exam
Authoritative Data Verified Data
amino acid ratio
take an appropriate amount of this product, add hydrochloric acid solution (1-2), and hydrolyze it at 110 ° C. For 24 hours, then determine it according to the appropriate amino acid analysis method. The relative ratio of each amino acid shall be calculated by dividing the sum of the moles of aspartic acid, glutamic acid, alanine, leucine, isoleucine and lysine by 19 as 1, and shall meet the following requirements: aspartic acid 3.4~4.6, glutamic acid 5.4~6.6, serine 2.4~3.6, threonine 2.4~3.6, alanine 2.7~3.3, valine 2.4~3.6, leucine 0.8~1.2, isoleucine 0.8~1.2, lysine 3.4~4.6.
clarity and color of solution
take an appropriate amount of this product, add (0.02mOl / L phosphate buffer (pH 7.0) (take potassium dihydrogen phosphate 2.72g, add O. 116.4ml of 1 mol/L sodium hydroxide solution was dissolved, diluted to 0901 ml with water), dissolved and diluted to prepare a solution containing 0902 mg per 1 ml, which was checked according to law (General rules 1 and 1), the solution should be clear and colorless. If it is turbid, it should not be more concentrated compared with No. 1 turbidity standard solution.
acetic acid
take an appropriate amount of this product, weigh it accurately, add diluent [0.02mol/L phosphate buffer (pH 7.0 )-methanol (95:5 ) ] dissolve and quantitatively dilute to prepare 2 per 1 ml. 0 mg of the solution was used as a test solution. The content of acetic acid should not exceed 0872 as determined by the acetic acid assay in synthetic polypeptides (General rule 5.0%).
Related substances
take an appropriate amount of this product, add 0.02mol/L phosphate buffer (pH 7.0) to dissolve and dilute to prepare a solution containing 0.5mg thymalfasin per 1 ml as a test solution; 2ml was accurately measured and placed in a 100ml measuring flask, diluted to the scale with the above buffer, and shaken to serve as a control solution. According to the chromatographic conditions under the content determination item, 20ul of each of the test solution and the control solution are accurately measured and injected into the liquid chromatograph respectively, and the chromatograms are recorded. If there are impurity peaks in the chromatogram of the test solution, except the solvent peak and the acetic acid peak, the area of a single impurity peak shall not be greater than the area of the main peak of the control solution (2.0% )9 The sum of each impurity peak area shall not be greater than 2 times (4.0%) of the main peak area of the control solution. The peaks in the chromatogram of the test solution which were 0.05 times smaller than the main peak area of the control solution were ignored.
moisture
take this product, according to the determination of moisture (General 0832 first method), the water content shall not exceed 5.0%.
THYMOSIN ALPHA 1 - Content determination
Authoritative Data Verified Data
measured by high performance liquid chromatography (General 0512).
chromatographic conditions and system suitability test
silica gel bonded with eighteen alkyl silane was used as filler; Ammonium sulfate buffer (26.4g of ammonium sulfate, 25ml of phosphoric acid, dissolved in water and diluted to 2000ml)-acetonitrile (90:10) as mobile phase A, the mobile phase B was ammonium sulfate buffer-acetonitrile (50:50); The column temperature was 50 ° C.; The detection wavelength was 210nm; And the gradient elution was performed according to the following table. Take appropriate amounts of the new thymic method reference and impurity work reference, add 0.02mol/L squamous acid salt buffer (pH 7.0) to dissolve and dilute to make a mixed solution containing 0.5mg thymic method and impurity I 50ug per 1 ml, 20u1 was injected into the liquid chromatograph, and the proportion of mobile phase B at 45 minutes of the elution gradient table was adjusted so that the retention time of thymalfasin was about 30 minutes, and the chromatogram was recorded. The number of theoretical plates shall not be less than 2000 calculated by thymalfasin peak, and the separation degree between impurity I peak and thymalfasin peak shall be greater than 1.2.
assay
take an appropriate amount of this product, precision weighing, plus 0.02mol/L phosphate buffer (pH 7.0) dissolved and quantitatively diluted to make a solution containing 0.5mg of thymalfasin per lml, as a test solution; 20ul of the test solution was injected into the liquid chromatograph with precision, and the chromatogram was recorded. An appropriate amount of new thymic method reference was taken and determined by the same method. According to the external standard method to calculate the peak area, that is.
THYMOSIN ALPHA 1 - Category
Authoritative Data Verified Data
immunomodulatory drugs.
THYMOSIN ALPHA 1 - Storage
Authoritative Data Verified Data
protected from light, sealed and stored at ~ 8°C.
THYMOSIN ALPHA 1 - Thymosin α1 for Injection
Authoritative Data Verified Data
This product is a sterile freeze-dried product made by adding a proper amount of excipients to the thymus gland. Containing thymalfasin (C129H215N33055) shall be between 90.0% and 110.0% of the labeled amount.
trait
This product is white or white loose block.
identification
take this product, each add water lml dissolved, according to the thymic method under the new item identification test, showed the same results.
examination
- pH value: take this product, add 5ml of water to dissolve each branch, and measure it according to law (General rule 0631). The pH value should be 6.0~7.5.
- the clarity and color of the solution shall be taken, and each one shall be dissolved by 1 ml of added water, which shall be checked according to law (General Principles 0902 and 0901). The solution shall be clear and colorless; If it is turbid, compared with No. 1 turbidity standard solution, should not be more concentrated. The content uniformity of
- shall be calculated based on the content of each bottle measured under the content determination item, and shall comply with the regulations (General rule 0941).
- Related substances take this product, add 0.02mol/L phosphate buffer (pH 7.0) to dissolve and dilute to prepare a solution containing 0.5mg thymalfasin per 1 ml as a test solution, according to the method under the new item of thymic method determination, should comply with the provisions.
- take an appropriate amount of moisture and determine the moisture content according to the method of determination of moisture content (General rule 0832, first method). The moisture content shall not exceed
- bacterial endotoxin take this product, according to the law to check (General 1143), each 1 mg of thymalfasin containing endotoxin should be less than 10EU.
- others should comply with the relevant provisions under injection (General 0102).
Content determination
Take 10 bottles of this product, and add 3ml of 0.02mol/L phosphate buffer (pH 7.0) into each bottle to dissolve it as a test solution, and determine it according to the new method of thymic method, that's right.
category
Same as thymalfasin.
specification
1.6mg
storage
It was sealed and stored at ~ 8°C.
THYMOSIN ALPHA 1 - Reference Information
| Overview | Thymosin alpha 1 (thymosin alpha 1) is a drug peptide that treats chronic hepatitis B and enhances immune system reactivity. |
| existence | Thymosin α1 is a highly conserved acid polypeptide structure, mainly distributed in the thymus tissue, in particular, thymic epithelial cells are also present in lymphoid tissues (e. G., spleen, lymph nodes) and non-lymphoid tissues (e. G., lung, kidney, and brain), its secretion and release are not regulated by other hormones or releasing factors. |
| Related studies | in vivo studies, thymosin alpha 1 can increase the expression level of interleukin 2 receptor in lymphocytes of mice activated by concanavalin A, and increase the secretion level of interleukin 2. |
| Application | Thymosin alpha 1 is an immunomodulatory compound that enhances the immune response to Thl. Thymosin alpha 1 has immunomodulatory effect on patients with immunodeficiency, so that Thymosin Alpha 1 has been widely used in clinical practice. |
| biological activity | Thymalfasin (thymosin-alpha 1) is an immunomodulatory compound that enhances the immune response to Thl. |
Supplier List
CAS: 62304-98-7
Tel: +86-17551318830
Email: r@reformchem.com
Mobile: +86-17551318830
QQ: 3785839865
CAS: 62304-98-7
Tel: 17505222756
Email: pules.cn@gmail.com
Mobile: +86-17551318830
Wechat: 17505222756
CAS: 62304-98-7
Tel: 18301782025
Email: 3008007409@qq.com
Mobile: 18021002903
QQ: 3008007409
Wechat: 18301782025
CAS: 62304-98-7
Tel: +86-21-56795779
Email: charles7788@worldyachem.com
Mobile: +86-13651600618
QQ: 2850607228
Wechat: 13651600618
WhatsApp: +8613651600618
CAS: 62304-98-7
Tel: +86-17551318830
Email: r@reformchem.com
Mobile: +86-17551318830
QQ: 3785839865
CAS: 62304-98-7
Tel: 17505222756
Email: pules.cn@gmail.com
Mobile: +86-17551318830
Wechat: 17505222756
CAS: 62304-98-7
Tel: 18301782025
Email: 3008007409@qq.com
Mobile: 18021002903
QQ: 3008007409
Wechat: 18301782025
CAS: 62304-98-7
Tel: +86-21-56795779
Email: charles7788@worldyachem.com
Mobile: +86-13651600618
QQ: 2850607228
Wechat: 13651600618
WhatsApp: +8613651600618



